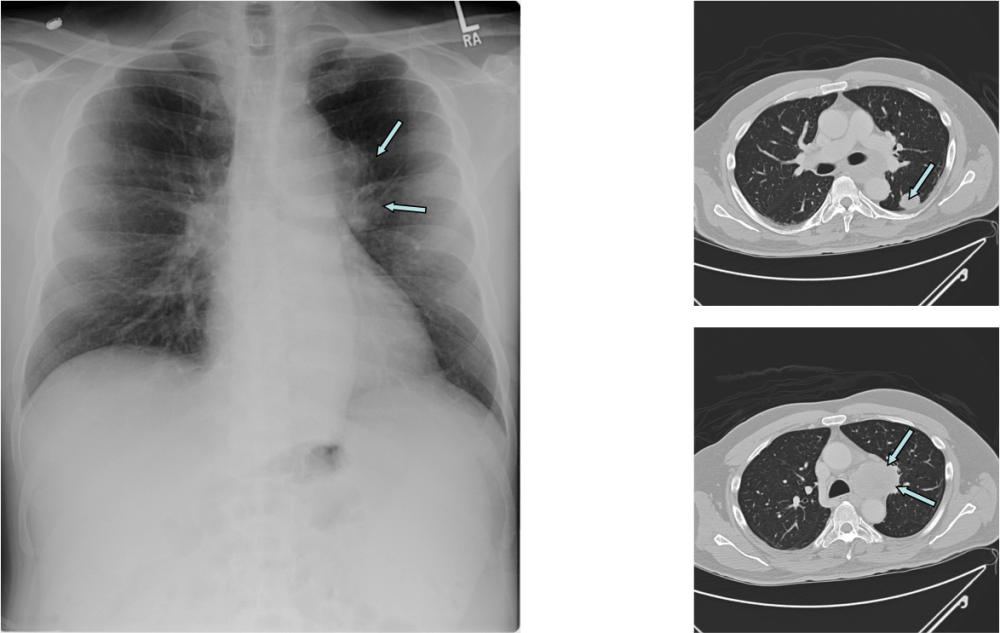
As history and electrodiagnostic tests were suggestive for myasthenic syndrome, he underwent serological testing for that condition: Ca channel P/Q antibody: 2.16 nmol/L (normal < 0.02), Ca channel N antibody: 0.05 nmol/L (normal < 0.02). Imaging studies showed two mass lesions, biopsy of one of which revealed small-cell lung carcinoma (SCLC). The patient underwent chemotherapy and has undergone remission of SCLC (he remains in remission for the last 8 years). His antibody titers have significantly declined but not back to normal. He remains symptomatic and is on 3,4 diaminopyridine 10 mg TID.
LEMS is a neuromuscular autoimmune disease, muscle weakness is caused by autoantibodies directed against P/Q voltage-gated calcium channels (VGCC) present on the presynaptic nerve terminal. Fifty- 60% of patients with LEMS have an associated tumor, usually SCLC, which also expresses functional VGCC. On the other hand, LEMS occurs in only 2-3% of SCLC patients, and it maybe under recognized. Lymphoma is another associated malignancy, although far less commonly encountered . LEMS in the remaining 40-50% of cases is a primarily autoimmune disease (i.e. non-tumor LEMS: NT-LEMS). There is an increase in susceptibility to autoimmune diseases in patients with NT-LEMS and their family members. Diagnosis of LEMS is ascertained on the basis of clinical symptoms and is further supported by electrophysiological and serological tests.
The clinical triad typically consists of proximal muscle weakness, autonomic features, and areflexia; although not a true apraxia, there is a functional gait impairment in LEMS patients which is out of proportion to what is expected from assessment of the muscles with manual motor testing 1. Proximal leg muscle weakness is usually the first symptom noted by the patient. A clinical suspicion of LEMS should be confirmed by electromyography, specially with the finding of a low amplitude of compound muscle action potential (CMAP) at rest without other findings which suggest a neuropathy such as abnormal sensory responses, prolonged latencies or slow conduction velocities. A screening test during the EMG study is to reassess the CMAP after a brief, 10 seconds period of maximal strength isometric exercise of the muscle CMAP is recorded from; An increment of CMAP amplitude >100% strongly suggests LEMS (but not 100% specific as it can happen in some cases of botulism as well as MG). Repetitive nerve stimulation (RNS) is then employed for confirming the diagnosis: low-frequency (2–5 Hz) RNS shows a decrement of CMAP amplitude by at least 10% 2. On the other hand, there is marked facilitation of the CMAP amplitude with high frequency stimulation (20-50 Hz), at least 25% but characteristically >100% 2. LEMS can be diagnosed by single-fiber electromyography(SFEMG). Stimulated SFEMG could be more sensitive than RNS and might even distinguish between LEMS and MG when measuring the neuromuscular jitter at different stimulation frequencies 3. Needle EMG can be misleading as it may demonstrate spontaneous activity and myopathic units / recruitment.
Antibodies to P/Q-type VGCC are responsible for the clinical symptoms of LEMS. These antibodies have been detected in 85–90% of patients with LEMS, and some studies report a percentage close to 100% in LEMS patients with SCLC. Antibodies to SOX1 (SRY-related HMG box 1) represent an additional potential diagnostic marker for LEMS as they have high specificity for SCLC 4. Once LEMS is diagnosed, screening for a possible associated tumor should be started immediately so CT and PET is recommended. Survival of patients with SCLC who also have vs those who do not have LEMS, which is in contrast to the other paraneoplastic manifestations of SCLC, which generally have a poor prognosis 5. This survival advantage may be due to serum factors such as P/Q VGCC antibodies or other soluble components reducing SCLC tumor cell as a result of cell-surface binding to VGCCs 5.
References:
1. Nicolle MW. Myasthenia Gravis and Lambert-Eaton Myasthenic Syndrome. Continuum 2016;22:1978-2005.
2. Medicine AQACAAoE. Literature review of the usefulness of repetitive nerve stimulation and single fiber EMG in the electrodiagnostic evaluation of patients with suspected myasthenia gravis or Lambert-Eaton myasthenic syndrome. Muscle & nerve 2001;24:1239-1247.
3. Chaudhry V, Watson DF, Bird SJ, Cornblath DR. Stimulated single-fiber electromyography in Lambert-Eaton myasthenic syndrome. Muscle & nerve 1991;14:1227-1230.
4. Sabater L, Titulaer M, Saiz A, Verschuuren J, Gure AO, Graus F. SOX1 antibodies are markers of paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology 2008;70:924-928.
5. Maddison P, Gozzard P, Grainge MJ, Lang B. Long-term survival in paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology 2017;88:1334-1339.